How do you find the protons, neutrons, and electrons for a Bromine (#1^-#) anion with a mass number of 80?
Bromine Mass Number
Drag and drop countries around the map to compare their relative size. Is Greenland really as big as all of Africa? You may be surprised at what you find! A great tool for educators. Bromine, isotope of mass 80 (80Br)Hydrogen bromide. 3 Chemical and Physical Properties. 3.1 Computed Properties. Property Name Property Value Reference; Molecular Weight: 80.9265 g/mol: Computed by PubChem 2.1 (PubChem release 2019.06.18). Today, bromine is primarily obtained by treating brines from wells in Michigan and Arkansas with chlorine. Elemental bromine is a hazardous material. It causes severe burns when it comes in contact with the skin and its vapor irritates the eyes, nose and throat.
1 Answer
The atomic number is the number of Protons add one for the number of electrons and 80 - 35 is the number of neutrons.
Explanation:
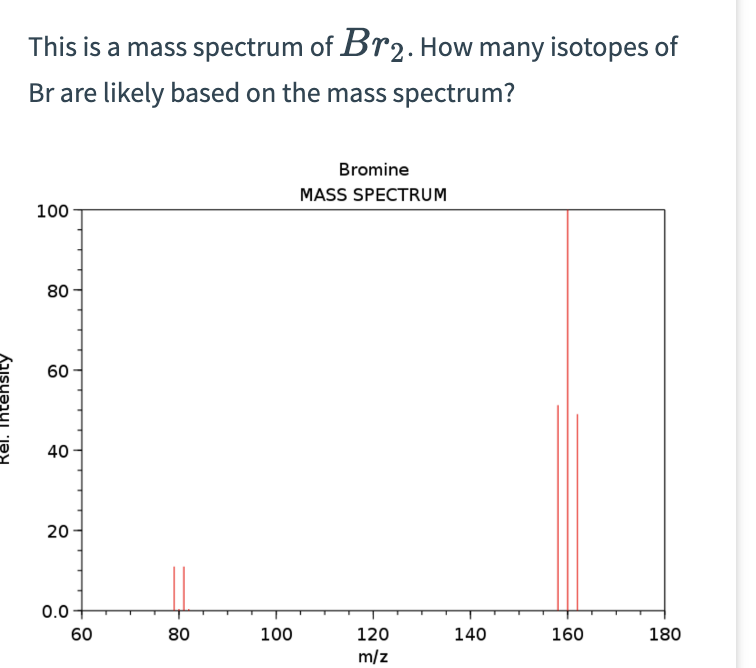
Bromine Mass Spec Images

The atomic number is the number of protons. This is the number that never changes for an element (or else the chemical properties will change!).
For Bromine the atomic number is 35. so number of protons = 35
In a neutral atom the number of protons = the number of electrons. 35 protons = 35 electrons. But Bromine anion with a charge of -1 has one extra electron so 35 +1 = 36 electrons.
36 electrons is the number of electrons in the stable inert gas Krypton. (That's why ions are more stable than atoms and atoms tend to form ions.)
The atomic mass is the sum of the number of protons and the number of neutrons (as electrons weigh almost nothing) so
Protons + neutrons = Mass putting in known values
n = 45

So Bromine
Related questions
